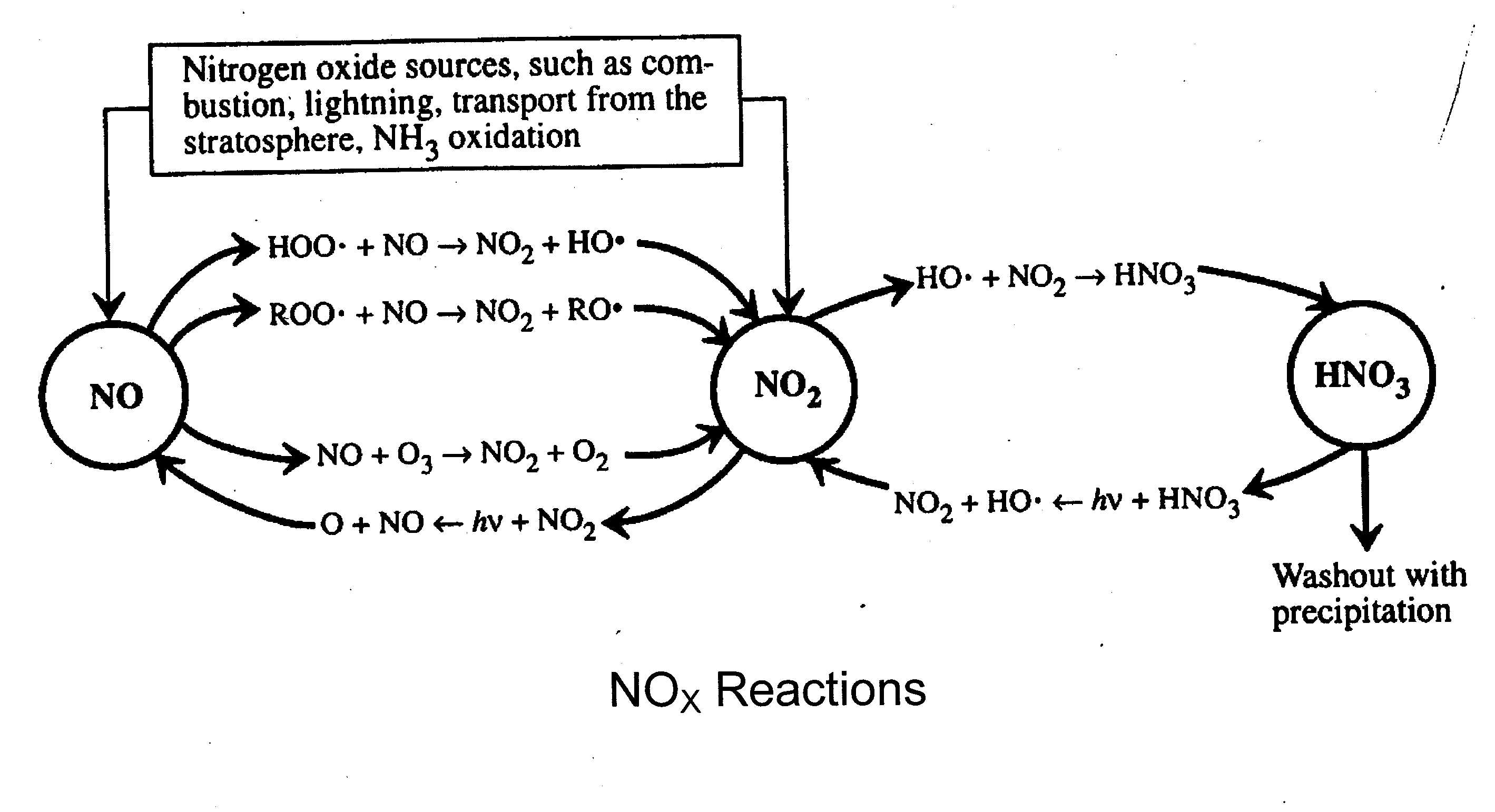
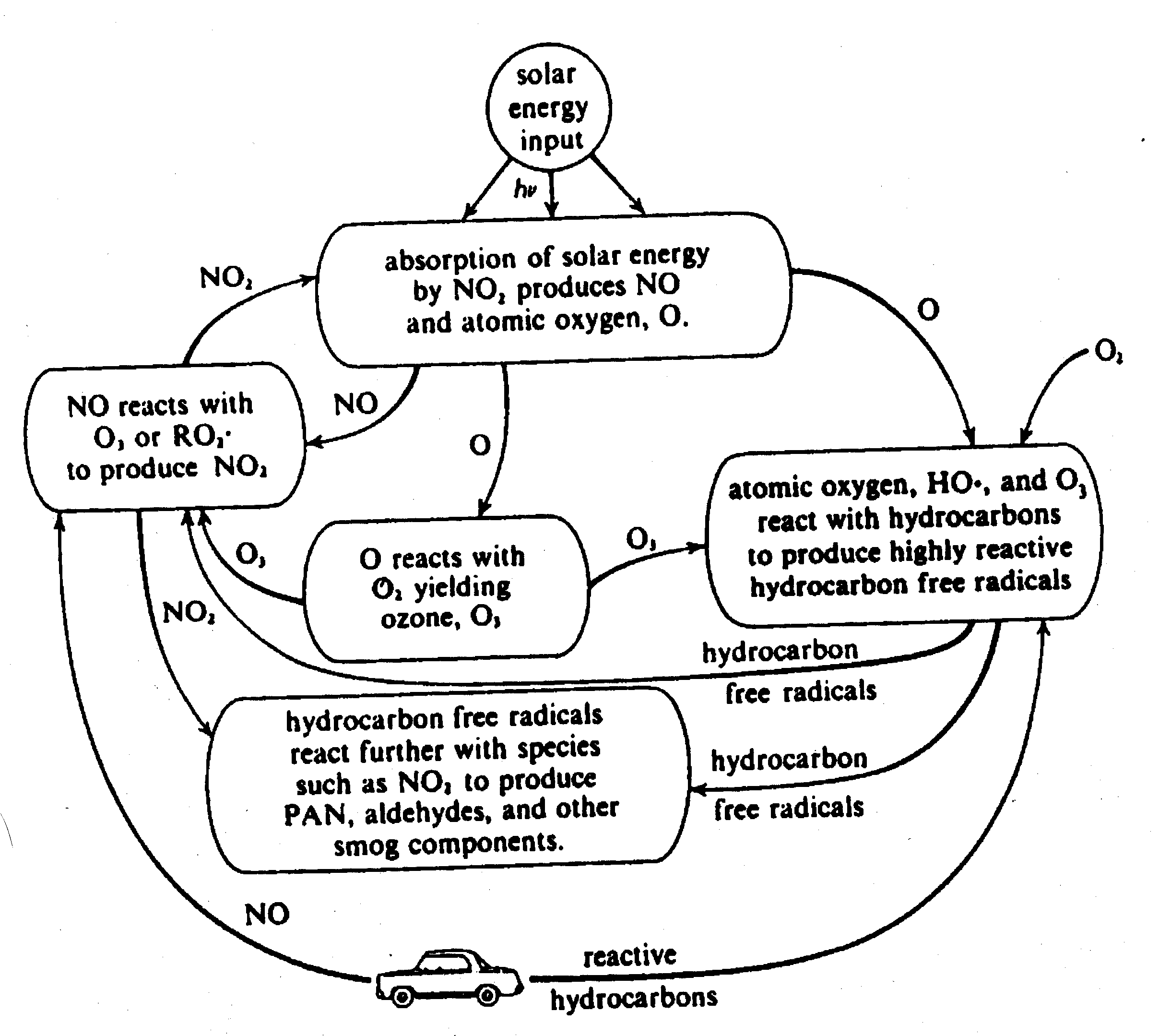
Notes on Smog and
Acid Rain
Oxidation States:
-
Atoms of a free element have oxidation state of 0
-
Total of all oxidation states must add up to charge on the species
-
Group 1 metals have oxidation numbers of +1 in their compounds
-
Group 2 metals have oxidation numbers of +2
-
Fluorine in compounds is +1
-
Hydrogen in compounds is +1 (except when bound to metals in hydrides,
e.g. LiH, where it is -1)
-
Oxygen is -2 (except in peroxides, e.g. H2O2, where it is -1)
Return to Chapter Page


