Week 1 class notes
Environmental Chemistry includes:
Basic cell processes
Nutrients needed and wastes produced
Basic toxicology
Water Chemistry
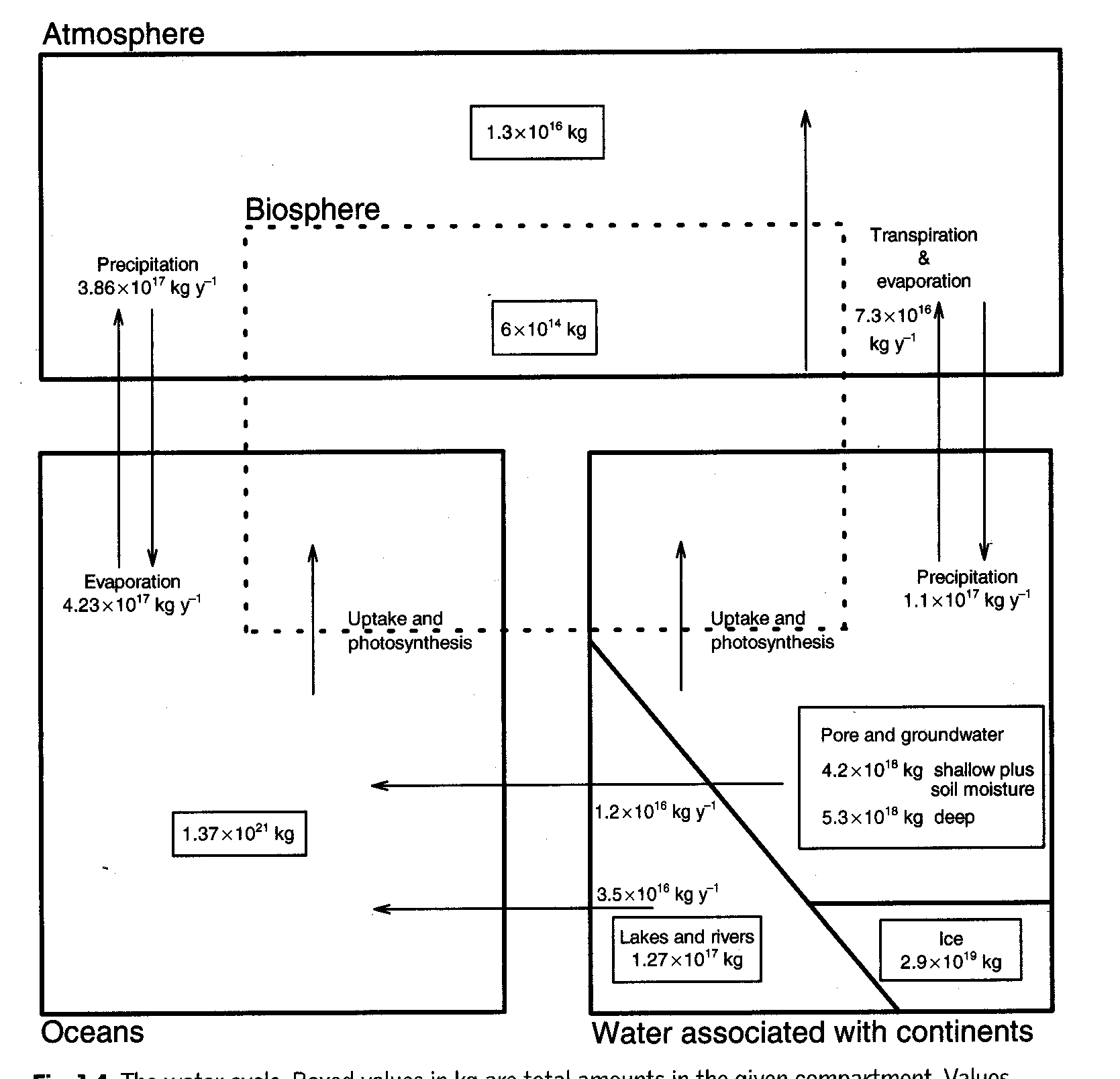
Hydrologic Cycle
Cycle of water from land and surface water (evaporation and transpiration) to atmosphere and back by precipitation.

Amount of precipitation is very variable
In US:
Properties of water
Stratification due to temperature:
Arises from density changes with temperature. Water’s density increases as temperature goes down -- until 4oC. Then it becomes less dense, and freezes at 0 oC. How does this affect a deep body of water?
The epilimnion: Upper layer--warmed by sun--well oxygenated--lighter because it is warmer.
The hypolimnion: Deeper water layer--cooler--low in oxygen--may be nutrient rich from exchange with sediments.
The thermocline is the border between these regions.
Aquatic Chemistry
Acid-base reactions:
In water we can define acids as substances which generate H+ in solution.
Bases generate OH-.
Water is both: H2O ® H+ + OH-
The equilibrium constant for this reaction is:
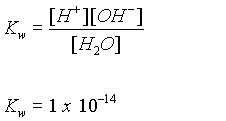
but [H2O] is considered to be a constant and is combined into the Kw.
Calculate the pH of pure water:
CO2 and H2O interact in important ways to control the pH and buffer changes in pH of water:
Examples:
CO2 + H2O Û H2CO3
H2CO3 Û H+ + HCO3-
HCO3- Û H+ + CO32-
Identify: Carbonate ion
Carbonic acid
Hydrogen carbonate ion (bicarbonate ion)
Other phenomena which happen in water:
Oxidation is the loss of electrons:
Fe ® Fe3+ + 3 e-
Oxidation can only take place if a reduction (gain of electrons) also takes place.
O2 + 2H+ + 4 e-® 2 OH-
Then the ions formed may react:
Fe3+ + 3 OH- ® Fe(OH)3 (insoluble iron oxide)
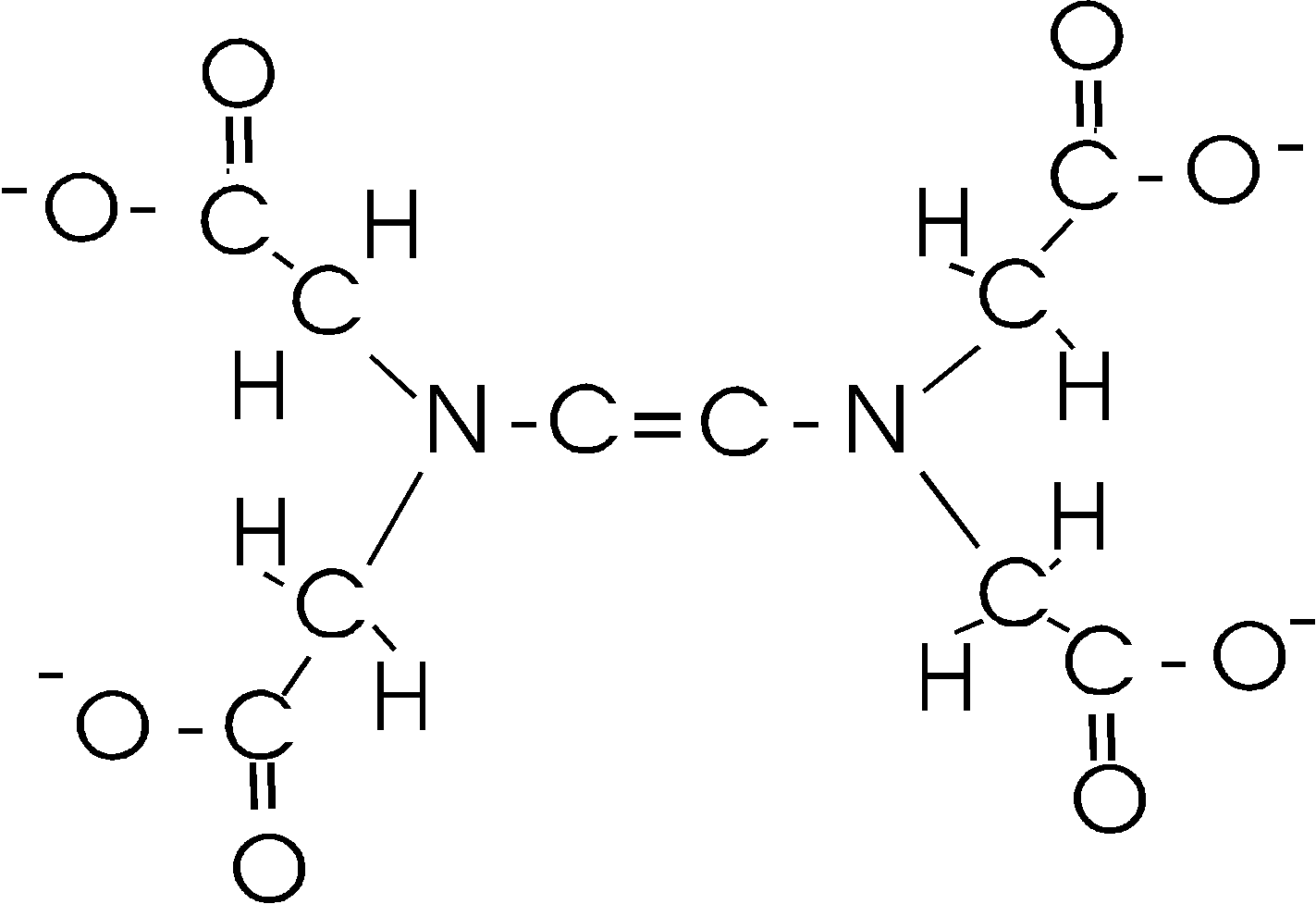 EDTA
EDTA
The Atmosphere
Air contains:
78.1% N2, 21.0% O2, 0.9% Ar, 0.03% CO2
Variable amounts of water vapor (1- 3%, usually)
Trace gases (Ne, CH4, Kr, Nox, Sox, O3, CO, NH3, etc.)
types of chemistry in the atmosphere
Absorption of light of the proper wavelength (i.e. energy) can break chemical bonds
O2 + hn ® O + O
The O radicals are very reactive and immediately do something--like
O + O2 + M ® O3 + M forming ozone.
(M is a particle surface used to hold the atoms together long enough to react. Some reactions will not happen at all without such an assistance--others will happen much less frequently )
NO or N2O may be oxidized to N2O5 which reacts with water droplets to form nitric acid.
N2O5 + H2O ® 2 HNO3
Organic gases in the air, (methane, propane etc.) can be oxidized, especially by the O radicals formed in photolysis of O2, making reactive organic species which can polymerize into larger molecules and eventually form visible particles -- smog.
Particulate matter in the atmosphere
dispersion aerosols
condensation aerosols
Most of the effects of particulates in the atmosphere are surface effects and so smallest particles have largest surface / mass ratio and greater effects than larger particles. These are least likely to settle out and are also hardest to contain in scrubbers.
The Geosphere
The Biosphere
CO2 + H2O ® {CH2O} + O2 (g)
Also organisms contribute to the breakdown of organic matter either with O2 or without:
{CH2O} + O2 (g) ® CO2 + H2O (aerobic)
or
2 {CH2O} ® CO2 (g)+ CH4 (anaerobic)
Cycles of Matter
Matter is continually cycled among all the areas of the environment:
Interchange of Materials
| From ¯ To® | Atmosphere | Hydrosphere | Biosphere | Geosphere | Anthrosphere |
Atmo-sphere | ----
| Precipitation | O2, CO2 | Particulates | O2, N2 other gases extracted |
Hydro-sphere | Water,
salt spray
| ---- | water | minerals sediments | Water, H2, O2 |
Biosphere | O2, CO2 | Nutrients | ---- | Organic matter | Food, materials etc etc |
Geosphere | Particles, volcanic gases | Soluble ions | Mineral nutrients | ---- | Fuel, minerals |
Anthro-sphere | Air pollutants | Water pollutants | Fertilizers | Landfills | ---- |
Review of Chemical Principles on Equilibrium
For the general reaction:
aA + bB ® cC the reaction
cC ® aA + bB also takes place
the quantity 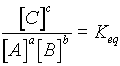 is a constant when the reaction is at equilibrium.
is a constant when the reaction is at equilibrium.
At equilibrium the rate of the forward reaction is equal to that of the reverse reaction.
Equilibrium position related to magnitude of Keq.
Relation of rate of reaction (Kinetics) to reaction constant (equilibrium)
Oxygen in Water
** Rate of dissolution depends strongly on contact and amount of surface between air and water.
** Rate of consumption depends on reactions in water which use O2.
{CH2O} + O2 ® CO2 + H2O
(degradation of organic material)
What is the qualitative effect of raising temperature on O2 concentration in water?
Acidity--Capability to neutralize OH-
Alkalinity--Capability to neutralize H+
pH -- Expresses concentration of H+
(and also OH-
from Kw
Strong acids (free mineral acids)
Hydrochloric HCl
Nitric HNO3
Sulfuric H2SO4 All totally ionize in water--
Weak acids
Acetic and other carboxylic acids ---COOH group
Carbonic acid H2CO3
Acidity of some hydrated metals:
Al(H2O)63+ Û Al(H2O)5OH2+ + H+
Buffers