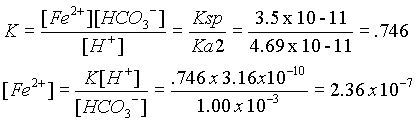
1. HOAc ® H+ + OAc-
H2O + H+ ® H3O+
Fe2+ + Fe3+ + e
H+ + e ® ½ H2
Redox reactions require a shift of electrons from a donor (reducing agent) to a receptor (oxidizing agent), Acid base reactions, in water at least, consist of the transfer of a proton (H+) from an acid (proton donor) to a base (proton acceptor).
2. The stable Fe+2 solid is either Fe(OH)2 or FeCO3. The more insoluble one, that is the one which gives a lower Fe+2 concentration for the solubility calculation will be the predominant species.
For FeCO3, we need the dissociation constant for HCO3- (Ka2 = 4.69 x 10 -11), and the Ksp (3.5 x 10 -11) and the [H+] from the pH.
The reaction is:
FeCO3 + H+ ® Fe+2 + HCO3-
Find the K for the above reaction by combining Ksp and Ka2 reactions.
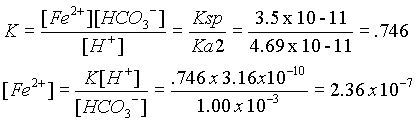
For Fe(OH)2,
the dissolution reaction is
Fe(OH)2 + 2H+ ® Fe+2 + 2H2O
Ksp = [Fe+2] / [H+]2 = 8 x 10-12
[Fe+2] = 8 x 10-7
Since FeCO3 is less soluble under these conditions, it will predominate.
5.
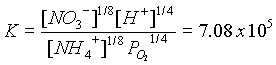 (see Sec. 4.8)
(see Sec. 4.8)
NO3-and NH4+ cancel because they are equal.
H+ is calculated from pH as 1 x 10-7. PO2 = 3.99 x 10 -31
7. Use Eqn 4.9.4, pE = 14.58
10. Use Eqn 4.11.13. Ksp' = 9.1 x 103, [Fe+2] = 3.79 x 10-4
12. Removes necessity of accounting for number of electrons in calculations, and balancing equations. Makes free energy values easier to compare.